Difference between revisions of "Cloud Maker -- Adiabatic Expansion"
From UO Physics Demonstration Catalog
(Created page with "{{NewDemo|subject=Thermodynamics|topic=Heat and the First Law|file1=Adiabatic_Clouds.gif}} A small amount of smoke from a burning match is introduced into the flask and the air is inside is saturated with water vapor by shaking the water inside. Pressing the bulb compresses the air in the flask, and releasing it causes expansion and cooling, forming a cloud. '''Location: ''' * Shelf T-2") |
|||
| (One intermediate revision by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{NewDemo|subject=Thermodynamics|topic=Heat and the First Law|file1=Adiabatic_Clouds.gif}} | {{NewDemo|subject=Thermodynamics|topic=Heat and the First Law|file1=Adiabatic_Clouds.gif|file2=Cloud make 2.jpg|file3=Cloud make 1.jpg}} | ||
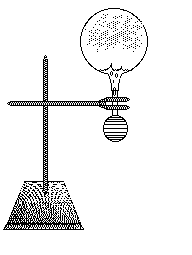
A small amount of smoke from a burning match is introduced into the flask and the | An upside down ball flask has a bulb pump filled with water attached to the bottom, with a glass stopper. A small amount of smoke from a burning match is introduced into the flask and the stopper with the bulb of water is attached. Squeezing the bulb puts water int the flask and increases the pressure in the glass. Releasing the bulb, the water is sucked out of the glass rapidly and the adiabatic expansion causes the air to cool and the water vapor inside condenses and makes a cloud. Having thee bulb illuminated can make the vapor more visible. | ||
'''Location: | '''Location: | ||
''' | ''' | ||
* Shelf T-2 | * Shelf T-2 | ||
* Lighter tool-box | |||
* Bicycle Pump | |||
* Lamp Q-3 | |||
* plug SH-130 | |||
Latest revision as of 09:24, 4 March 2024
Return to Heat and the First Law
Description:
An upside down ball flask has a bulb pump filled with water attached to the bottom, with a glass stopper. A small amount of smoke from a burning match is introduced into the flask and the stopper with the bulb of water is attached. Squeezing the bulb puts water int the flask and increases the pressure in the glass. Releasing the bulb, the water is sucked out of the glass rapidly and the adiabatic expansion causes the air to cool and the water vapor inside condenses and makes a cloud. Having thee bulb illuminated can make the vapor more visible.
Location:
- Shelf T-2
- Lighter tool-box
- Bicycle Pump
- Lamp Q-3
- plug SH-130