Sunset
From UO Physics Demonstration Catalog
Return to Color
Description:
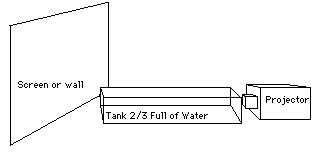
Fill 2/3 of the tank or a 200mL beaker with water. Add 150 mL. of a saturated solution of Sodium Thiosulfate. In class, The addition of 5-10 ml. of HCL will initiate the precipitation with Sulfur which will scatter the light.. The side of the tank shows the short wavelength blue part of the spectrum being scattered, and the light on the screen shows the longer wavelengths of yellow and then red as more particles are created. This shows why the sun is yellow and the sky is blue during the day, and as the sunsets , the light interacts with more particles in the air, and the sun turns red.
NOTE: You can also show in the early stages that the scattered blue light on the side is polarized.
Safety. Goggles and latex type gloves for handling acid and goggles. Lab coat suggested.
Location:
- Chemicals: Fume Hood
- Large Tank: Shelf M-1
- 2000mL beaker: Next to Fume Hood.