Difference between revisions of "Super Cooling Water"
From UO Physics Demonstration Catalog
(Created page with "{{NewDemo|subject=Thermodynamics|topic=Thermodynamics|file1=}} Supercooling water is the process of cooling distilled water beyond zero degrees Celsius until it reaches a critical point to start a phase change into ice. By changing the phase to ice, the temperature in the test tube has increased to zero. To set this demo up, fill the larger backer with ethanol to ~ 600 ml, and fill the test tube with distilled water about 2/5 of the way. Next, set up the labpro along wi...") |
|||
| Line 1: | Line 1: | ||
{{NewDemo|subject=Thermodynamics|topic=Thermodynamics|file1=}} | {{NewDemo|subject=Thermodynamics|topic=Thermodynamics|file1=Super_cooling_6.JPG|file2=Super_cooling_3.JPG}} | ||
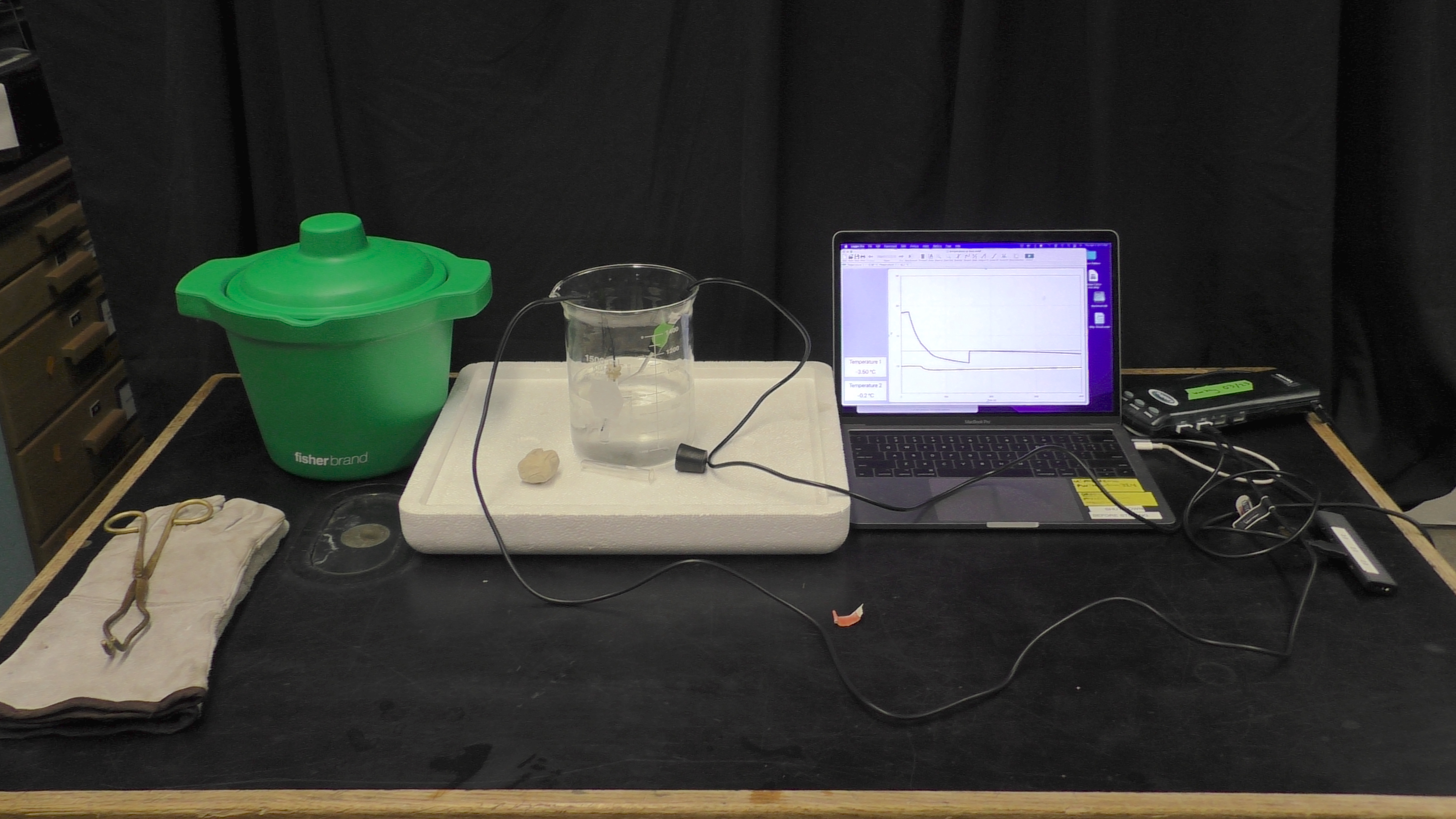
Supercooling water is the process of cooling distilled water beyond zero degrees Celsius until it reaches a critical point to start a phase change into ice. By changing the phase to ice, the temperature in the test tube has increased to zero. To set this demo up, fill the larger backer with ethanol to ~ 600 ml, and fill the test tube with distilled water about 2/5 of the way. Next, set up the labpro along with the temperature probe and the surface temperature probe. Place the surface temperature probe inside the test tube to measure the water temperature, and the temperate probe in the beaker to measure the alcohol reservoir. Cool the alcohol reservoir to about -10~-20 C by adding dry ice to meet this temperature. | Supercooling water is the process of cooling distilled water beyond zero degrees Celsius until it reaches a critical point to start a phase change into ice. By changing the phase to ice, the temperature in the test tube has increased to zero. To set this demo up, fill the larger backer with ethanol to ~ 600 ml, and fill the test tube with distilled water about 2/5 of the way. Next, set up the labpro along with the temperature probe and the surface temperature probe. Place the surface temperature probe inside the test tube to measure the water temperature, and the temperate probe in the beaker to measure the alcohol reservoir. Cool the alcohol reservoir to about -10~-20 C by adding dry ice to meet this temperature. | ||
Revision as of 11:26, 1 May 2024
Return to Thermodynamics
Description:
Supercooling water is the process of cooling distilled water beyond zero degrees Celsius until it reaches a critical point to start a phase change into ice. By changing the phase to ice, the temperature in the test tube has increased to zero. To set this demo up, fill the larger backer with ethanol to ~ 600 ml, and fill the test tube with distilled water about 2/5 of the way. Next, set up the labpro along with the temperature probe and the surface temperature probe. Place the surface temperature probe inside the test tube to measure the water temperature, and the temperate probe in the beaker to measure the alcohol reservoir. Cool the alcohol reservoir to about -10~-20 C by adding dry ice to meet this temperature.
Location: