Difference between revisions of "Spectral Tubes 2"
From UO Physics Demonstration Catalog
(Created page with "{{NewDemo|subject=Light and Optics|topic=Spectral Lines|file1=tubes_01.jpg|file2=tubes_02.jpg|file3=tubes_03.jpg|file4=tubes_04.jpg}} Gas filled tubes are excited with a 5000v source. Diffraction lens glasses are passed out to the students (there are both full size diffraction glasses and 1/2 size, depending on audience size). Students are instructed to look at a line source such as a fluorescent light through the lens. Hold the lens so as to obtain the white light colo...") |
|||
| Line 1: | Line 1: | ||
{{NewDemo|subject=Light and Optics|topic=Spectral Lines|file1=tubes_01.jpg|file2=tubes_02.jpg|file3=tubes_03.jpg|file4=tubes_04.jpg}} | {{NewDemo|subject=Light and Optics|topic=Spectral Lines|file1=tubes_01.jpg|file2=tubes_02.jpg|file3=tubes_03.jpg|file4=tubes_04.jpg}} | ||
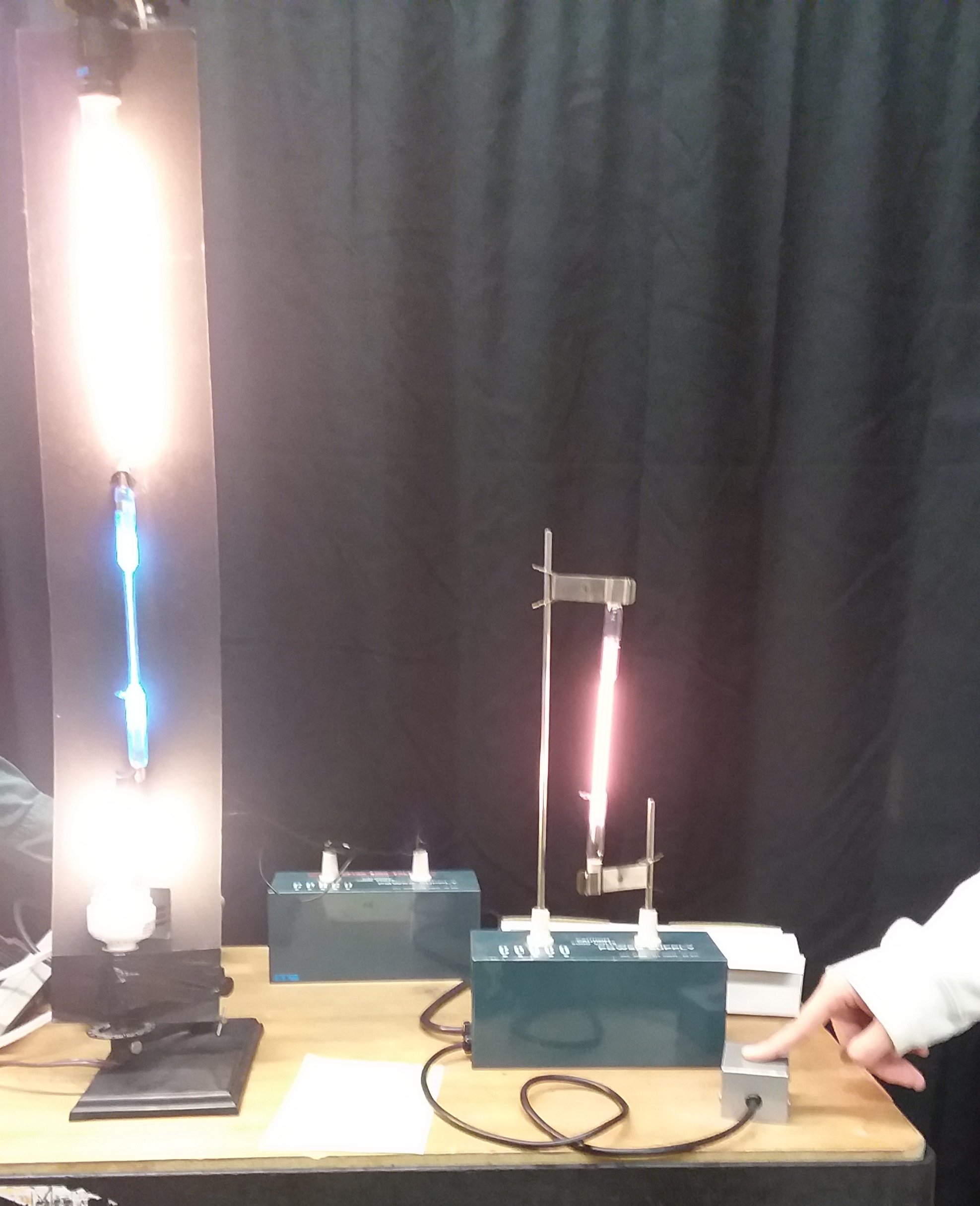
Gas filled tubes are excited with a 5000v source. Diffraction | '''Gas Emission Spectra:''' Gas filled tubes are excited with a 5000v source. Diffraction gratings are passed out to the students. Hold the grating very close to your eye to observe the color spectra that appears on both sides of the light source being observed. Students can observe the spectral lines obtained from the different gas tubes placed in the high voltage supply. (Spectrum Tube set-up is n the right side of the photo.) Hydrogen, Helium, Mercury, Neon and some other gasses are available. | ||
'''Other Emission Spectra. The Light Tower:''' Students are instructed to look the incandescent white light line source at the top of the light tower and observe the continuous light spectrum on both sides of the light source. Light the fluorescent bulb at the bottom and notice that the spectrum ins not continuous. Excite the mercury gas tube in the center and it can be observed that there are some spectral lines from the mercury that line up with the lines form the fluorescent bulb. This tells us there is mercury gas in the fluorescent tube. There are also some other lines which means ther is also another gas which commonly is Argon. Pictured in these photos: TOP Two is the incandescent light, fluorescent light, and the mercury gas tube. Bottom tube, the helium tube in the separate power supply is also excited. | |||
'''Location: | '''Location: | ||
Revision as of 20:28, 21 January 2023
Return to Spectral Lines
Description:
Gas Emission Spectra: Gas filled tubes are excited with a 5000v source. Diffraction gratings are passed out to the students. Hold the grating very close to your eye to observe the color spectra that appears on both sides of the light source being observed. Students can observe the spectral lines obtained from the different gas tubes placed in the high voltage supply. (Spectrum Tube set-up is n the right side of the photo.) Hydrogen, Helium, Mercury, Neon and some other gasses are available.
Other Emission Spectra. The Light Tower: Students are instructed to look the incandescent white light line source at the top of the light tower and observe the continuous light spectrum on both sides of the light source. Light the fluorescent bulb at the bottom and notice that the spectrum ins not continuous. Excite the mercury gas tube in the center and it can be observed that there are some spectral lines from the mercury that line up with the lines form the fluorescent bulb. This tells us there is mercury gas in the fluorescent tube. There are also some other lines which means ther is also another gas which commonly is Argon. Pictured in these photos: TOP Two is the incandescent light, fluorescent light, and the mercury gas tube. Bottom tube, the helium tube in the separate power supply is also excited.
Location:
- Spectral Tubes: Drawers 98 & 99
- Boxes of diffraction lenses: Sh. L-3
- Spectral Tube Power Supplies: Shelf L-3
- Spectral Tube holding stand: Sh. O-0 (on top of sh. O)