Difference between revisions of "Mechanical Equivalent of Heat"
From UO Physics Demonstration Catalog
(Created page with "{{NewDemo|subject=Thermodynamics|topic=Heat and the First Law|file1=Mechanical_equiv_of_Heat.gif}} A copper calorimeter is attatched to a crank. A rope is wrapped around the calorimeter and attatched to a 1 kg. mass. When the crank is turned the mass is raised a few centimeters off the floor and held there by the frictional force between the rope and calorimeter. The mechanical equivalent of heat can be calculated by determining the frictional work done by the calorimet...") |
|||
| Line 1: | Line 1: | ||
{{NewDemo|subject=Thermodynamics|topic=Heat and the First Law|file1=Mechanical_equiv_of_Heat.gif}} | {{NewDemo|subject=Thermodynamics|topic=Heat and the First Law|file1=Mechanical_equiv_of_Heat.gif}} | ||
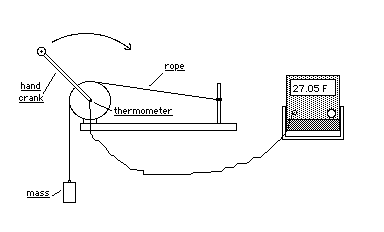
A copper calorimeter is | A copper calorimeter is attached to a crank. A rope is wrapped around the calorimeter and attached to a 1 kg. mass. When the crank is turned the mass is raised a few centimeters off the floor and held there by the frictional force between the rope and calorimeter. The mechanical equivalent of heat can be calculated by determining the frictional work done by the calorimeter and comparing it to the heat energy which raised the temperature of the calorimeter. | ||
'''Location: | '''Location: | ||
''' | ''' | ||
* Shelf F-2 | * Shelf F-2 | ||
Revision as of 14:09, 14 April 2022
Return to Heat and the First Law
Description:
A copper calorimeter is attached to a crank. A rope is wrapped around the calorimeter and attached to a 1 kg. mass. When the crank is turned the mass is raised a few centimeters off the floor and held there by the frictional force between the rope and calorimeter. The mechanical equivalent of heat can be calculated by determining the frictional work done by the calorimeter and comparing it to the heat energy which raised the temperature of the calorimeter.
Location:
- Shelf F-2