Difference between revisions of "Ruchardt's Tube"
From UO Physics Demonstration Catalog
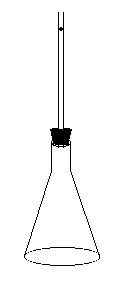
(Created page with "{{NewDemo|subject=Thermodynamics|topic=Ideal Gas Law|file1=Tube.GIF}} This demonstration measures the ratio Cp/Cv, the ratio of specific heat at constant pressure to specific heat at constant volume. The demo consists of a tube in a rubber stopper which fits into a flask. A metal ball is dropped into the tube and oscillates. By measuring the period of the oscillations, the ratio can be determined by using the equation [missing image], where a is the radius of the ball a...") |
|||
| Line 3: | Line 3: | ||
This demonstration measures the ratio Cp/Cv, the ratio of specific heat at constant pressure to specific heat at constant volume. The demo consists of a tube in a rubber stopper which fits into a flask. A metal ball is dropped into the tube and oscillates. By measuring the period of the oscillations, the ratio can be determined by using the equation [missing image], where a is the radius of the ball and P = Po + mg/a. | This demonstration measures the ratio Cp/Cv, the ratio of specific heat at constant pressure to specific heat at constant volume. The demo consists of a tube in a rubber stopper which fits into a flask. A metal ball is dropped into the tube and oscillates. By measuring the period of the oscillations, the ratio can be determined by using the equation [missing image], where a is the radius of the ball and P = Po + mg/a. | ||
Location: | '''Location: | ||
''' | |||
Shelf F-4 | * Shelf F-4 | ||
Latest revision as of 14:42, 14 April 2022
Return to Ideal Gas Law
Description:
This demonstration measures the ratio Cp/Cv, the ratio of specific heat at constant pressure to specific heat at constant volume. The demo consists of a tube in a rubber stopper which fits into a flask. A metal ball is dropped into the tube and oscillates. By measuring the period of the oscillations, the ratio can be determined by using the equation [missing image], where a is the radius of the ball and P = Po + mg/a.
Location:
- Shelf F-4