Difference between revisions of "Super Cooling Water"
From UO Physics Demonstration Catalog
| Line 1: | Line 1: | ||
{{NewDemo|subject=Thermodynamics|topic=Thermodynamics|file1=Super_cooling_6.JPG|file2=Super_cooling_3.JPG}} | {{NewDemo|subject=Thermodynamics|topic=Thermodynamics|file1=Super_cooling_6.JPG|file2=Super_cooling_3.JPG}} | ||
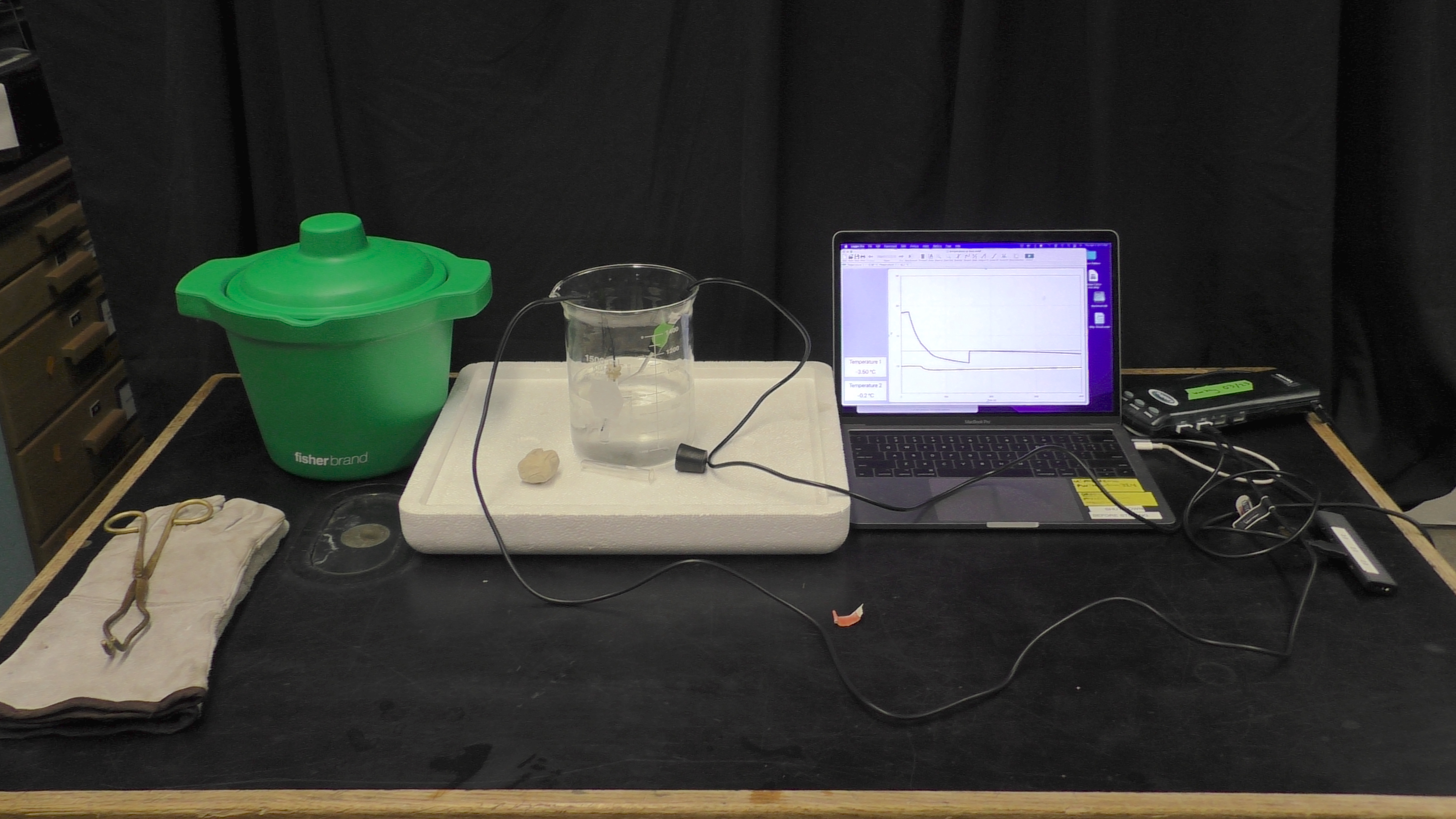
Super cooling water is the process of cooling distilled water beyond zero degrees Celsius until it reaches a critical point to start a phase change into ice. By changing the phase to ice, the temperature in the test tube has increased to zero. To set this demo up, fill the larger backer with ethanol to ~ 600 ml, and fill the test tube with distilled water about 2/5 of the way. Next, set up the labpro along with the temperature probe and the surface temperature probe. Place the surface temperature probe inside the test tube to measure the water temperature, and the temperate probe in the beaker to measure the alcohol reservoir. Cool the alcohol reservoir to about -10~-20 C by adding dry ice to meet this temperature. | |||
'''Location:''' | '''Location:''' | ||
* E-2 Styrofoam Bucket | * E-2: Styrofoam Bucket | ||
* | * F-4: Test tube from old set up | ||
* Fume Hood: Ethanol, and Distilled water | |||
* Glass ware: Beaker 1500ml | |||
* DR-46: Tongs | |||
* DR-42: Gloves | |||
* DR-142: Safety Glasses | |||
* G-2: Surface Temperature Sensor | |||
* G-2: Temperature Sensor | |||
Latest revision as of 13:56, 1 May 2024
Return to Thermodynamics
Description:
Super cooling water is the process of cooling distilled water beyond zero degrees Celsius until it reaches a critical point to start a phase change into ice. By changing the phase to ice, the temperature in the test tube has increased to zero. To set this demo up, fill the larger backer with ethanol to ~ 600 ml, and fill the test tube with distilled water about 2/5 of the way. Next, set up the labpro along with the temperature probe and the surface temperature probe. Place the surface temperature probe inside the test tube to measure the water temperature, and the temperate probe in the beaker to measure the alcohol reservoir. Cool the alcohol reservoir to about -10~-20 C by adding dry ice to meet this temperature.
Location:
- E-2: Styrofoam Bucket
- F-4: Test tube from old set up
- Fume Hood: Ethanol, and Distilled water
- Glass ware: Beaker 1500ml
- DR-46: Tongs
- DR-42: Gloves
- DR-142: Safety Glasses
- G-2: Surface Temperature Sensor
- G-2: Temperature Sensor