Difference between revisions of "Mechanical Equivalent of Heat"
From UO Physics Demonstration Catalog
m (Physdemo moved page Mechanical Equiv. of Heat to Mechanical Equivalent of Heat) |
|||
| Line 1: | Line 1: | ||
{{NewDemo|subject=Thermodynamics|topic=Heat and the First Law|file1=Mechanical_equiv_of_Heat.gif}} | {{NewDemo|subject=Thermodynamics|topic=Heat and the First Law|file1=Mechanical_equiv_of_Heat.gif}} | ||
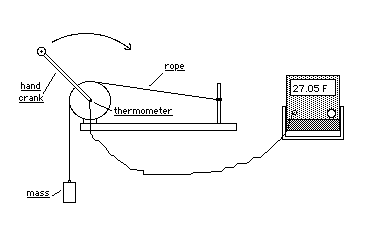
A copper calorimeter is attached to a crank. A | A copper calorimeter is attached to a crank. A rubber band is attached to a roperope is wrapped around the calorimeter and attached to a 1 kg. mass. When the crank is turned the mass is raised a few centimeters off the floor and held there by the frictional force between the rope and calorimeter. The mechanical equivalent of heat can be calculated by determining the frictional work done by the calorimeter and comparing it to the heat energy which raised the temperature of the calorimeter. | ||
'''Location: | '''Location: | ||
''' | ''' | ||
* Shelf F-2 | * Shelf F-2 | ||
Latest revision as of 08:10, 10 January 2023
Return to Heat and the First Law
Description:
A copper calorimeter is attached to a crank. A rubber band is attached to a roperope is wrapped around the calorimeter and attached to a 1 kg. mass. When the crank is turned the mass is raised a few centimeters off the floor and held there by the frictional force between the rope and calorimeter. The mechanical equivalent of heat can be calculated by determining the frictional work done by the calorimeter and comparing it to the heat energy which raised the temperature of the calorimeter.
Location:
- Shelf F-2