Difference between revisions of "Triple Point"
From UO Physics Demonstration Catalog
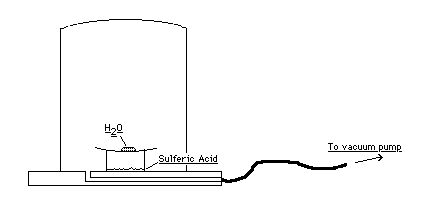
(Created page with "{{NewDemo|subject=Thermodynamics|topic=Thermal Properties of Matter|file1=Triple_Point.gif}} A small amount of water sits on a watch glass. The watch glass is placed on top of a specially cut pyrex beaker that contains a small amount of sulfuric acid to absorb water vapor. The set-up is placed inside a plexiglass vacuum chamber. The entire apparatus is placed on an overhead. When the vacuum pump is activated, the water boils. The boiling continues for a few minutes unti...") |
|||
| Line 1: | Line 1: | ||
{{NewDemo|subject=Thermodynamics|topic=Thermal Properties of Matter|file1=Triple_Point.gif}} | {{NewDemo|subject=Thermodynamics|topic=Thermal Properties of Matter|file1=Triple_Point.gif}} | ||
Older versions needs to be remade. | |||
A small amount of water sits on a watch glass. The watch glass is placed on top of a specially cut pyrex beaker that contains a small amount of sulfuric acid to absorb water vapor. The set-up is placed inside a plexiglass vacuum chamber. The entire apparatus is placed on an overhead. When the vacuum pump is activated, the water boils. The boiling continues for a few minutes until the water freezes. Often, a bubble trapped in the ice can be observed, i.e. at one time water exists in all three phases. | A small amount of water sits on a watch glass. The watch glass is placed on top of a specially cut pyrex beaker that contains a small amount of sulfuric acid to absorb water vapor. The set-up is placed inside a plexiglass vacuum chamber. The entire apparatus is placed on an overhead. When the vacuum pump is activated, the water boils. The boiling continues for a few minutes until the water freezes. Often, a bubble trapped in the ice can be observed, i.e. at one time water exists in all three phases. | ||
| Line 5: | Line 7: | ||
'''Location: | '''Location: | ||
''' | ''' | ||
* | * | ||
Latest revision as of 14:19, 9 January 2023
Return to Thermal Properties of Matter
Description:
Older versions needs to be remade.
A small amount of water sits on a watch glass. The watch glass is placed on top of a specially cut pyrex beaker that contains a small amount of sulfuric acid to absorb water vapor. The set-up is placed inside a plexiglass vacuum chamber. The entire apparatus is placed on an overhead. When the vacuum pump is activated, the water boils. The boiling continues for a few minutes until the water freezes. Often, a bubble trapped in the ice can be observed, i.e. at one time water exists in all three phases.
Location: