Difference between revisions of "Spectral Tubes 2"
From UO Physics Demonstration Catalog
(Added video showing spectrum lines) |
|||
| Line 11: | Line 11: | ||
* Spectral Tube Power Supplies: Shelf L-3 | * Spectral Tube Power Supplies: Shelf L-3 | ||
* Spectral Tube holding stand: Sh. O-0 (on top of sh. O) | * Spectral Tube holding stand: Sh. O-0 (on top of sh. O) | ||
[[File:Light Tower and Gas Spectrum.mp4|left|thumb]] | |||
Latest revision as of 14:16, 28 February 2024
Return to Spectral Lines
Description:
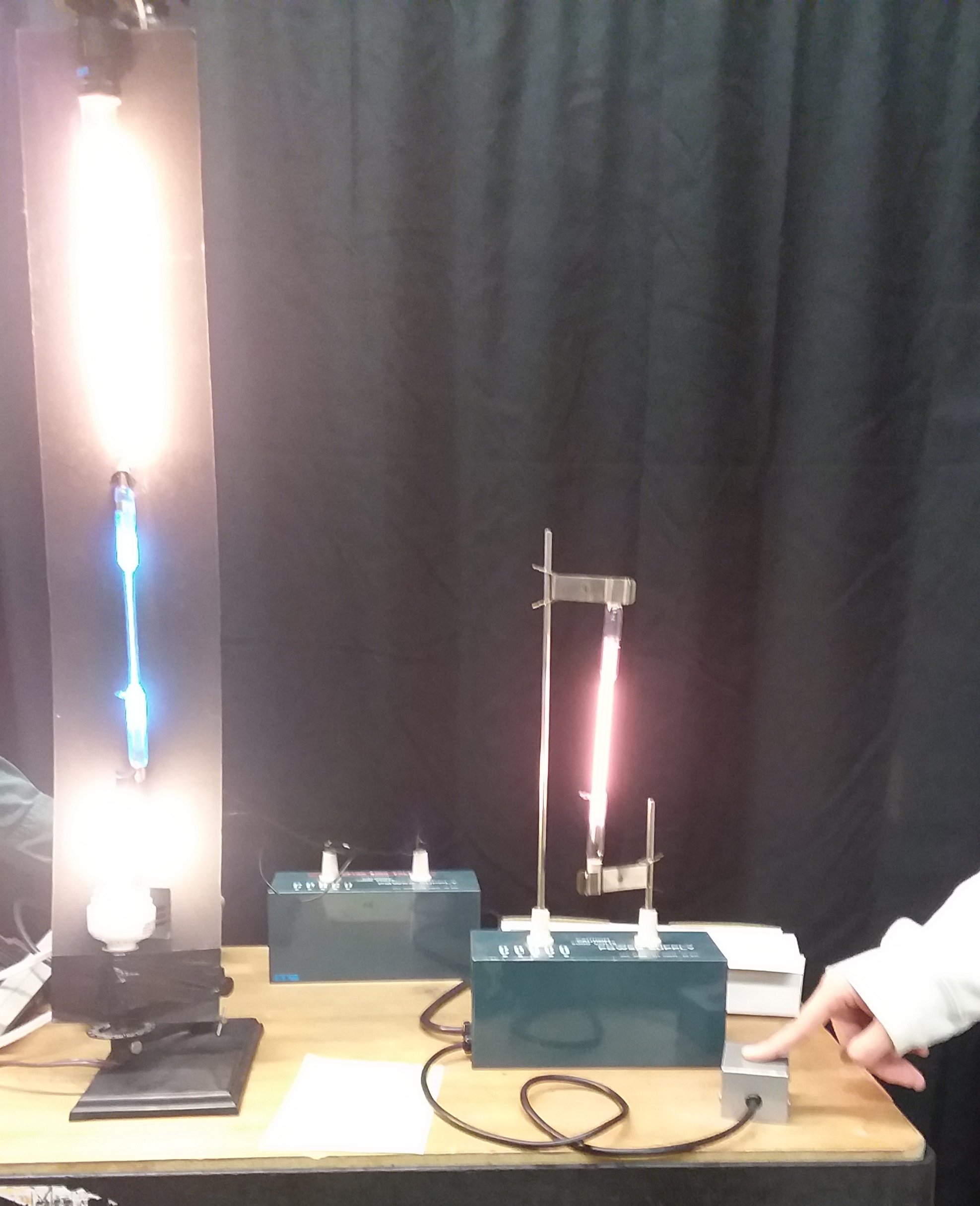
Gas Emission Spectra: Gas filled tubes are excited with a 5000v source. Diffraction gratings are passed out to the students. Hold the grating very close to your eye to observe the color spectra that appears on both sides of the light source being observed. Students can observe the spectral lines obtained from the different gas tubes placed in the high voltage supply. (Spectrum Tube set-up is n the right side of the photo.) Hydrogen, Helium, Mercury, Neon and some other gasses are available.
Other Emission Spectra. The Light Tower: Students are instructed to look the incandescent white light line source at the top of the light tower and observe the continuous light spectrum on both sides of the light source. Light the fluorescent bulb at the bottom and notice that the spectrum ins not continuous. Excite the mercury gas tube in the center and it can be observed that there are some spectral lines from the mercury that line up with the lines form the fluorescent bulb. This tells us there is mercury gas in the fluorescent tube. There are also some other lines which means ther is also another gas which commonly is Argon. Pictured in these photos: TOP Two is the incandescent light, fluorescent light, and the mercury gas tube. Bottom tube, the helium tube in the separate power supply is also excited.
Location:
- Spectral Tubes: Drawers 98 & 99
- Boxes of diffraction lenses: Sh. L-3
- Spectral Tube Power Supplies: Shelf L-3
- Spectral Tube holding stand: Sh. O-0 (on top of sh. O)